PRODUCT
OAβ test can be used for the early detection of Alzheimer’s Disease from a small blood sample.
Alzheimer's disease
Alzheimer’s Disease (“AD”) is perhaps the most significant neurodegenerative disease which accounts for roughly 70% of what is commonly referred to as dementia.
It is brought on gradually and progressively worsens. In 2015, there was a new dementia diagnosis every 3 seconds. In 2015, it is believed that a total of 150 million people will have dementia. Dementia, including AD, is #5 on the WHO list of “Top 10 Causes of Death,” and brings a tragic ending to so many individuals annually.
The need for early diagnosis

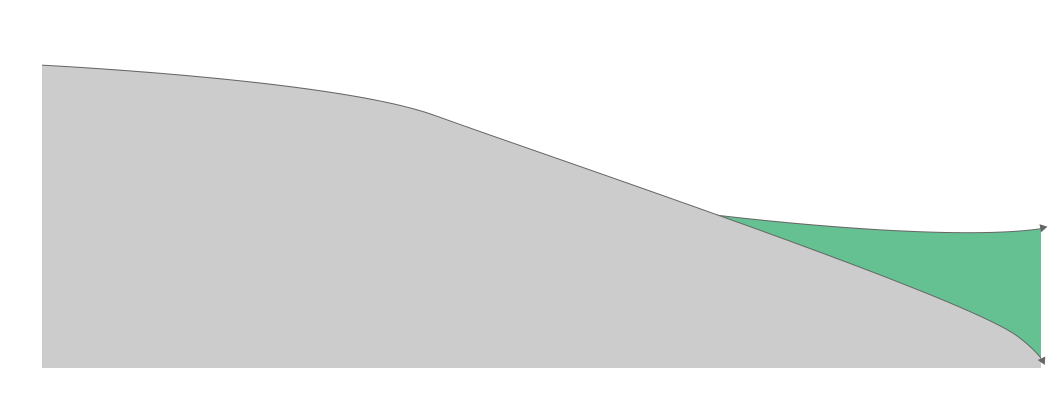


Due to the fact that there has still yet to be a cure for AD, and that lost cognitive function cannot be restored, the best way to manage AD is the delay of its onset through early diagnosis followed by preemptive care.
Early diagnosis biomarker: Beta-amyloid oligomers
-


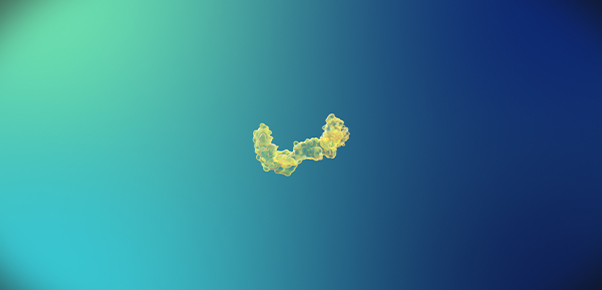 <Monomer>
<Monomer> -
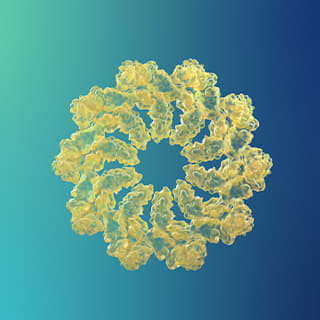

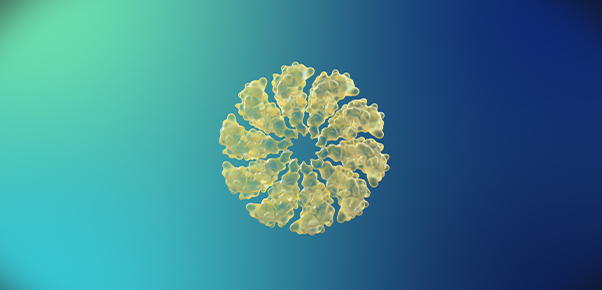 <Oligomers>
<Oligomers> -
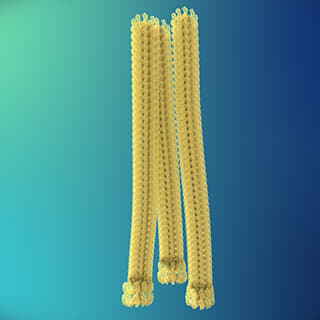

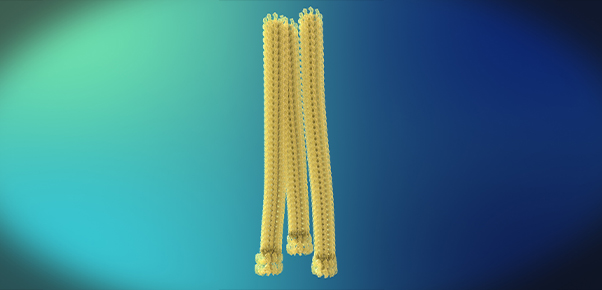 <Fibrills>
<Fibrills> -
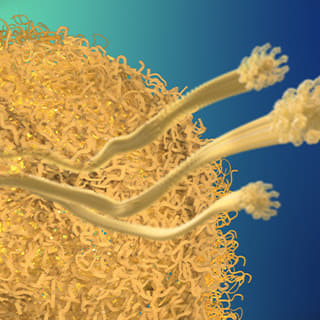

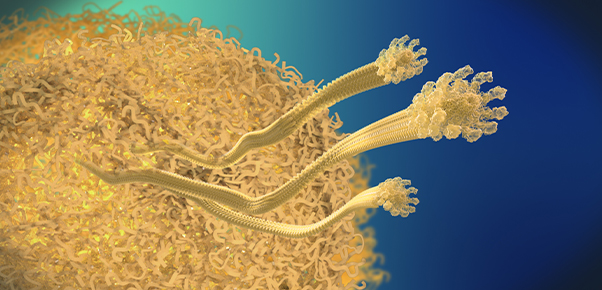 <Plaque>
<Plaque>
Beta-amyloid is known to be the main player in the pathology of AD. Proteins, such as beta-amyloid, that exist within the human body, can form aggregate compounds called oligomers. Beta-amyloid oligomers ultimately form insoluble amyloid plaque, which eventually causes brain damage, followed by AD.
OAβ Test:
The first ever AD blood test
OAβ Test from PeopleBio is the only blood test currently available that can measure the oligomerization levels of beta-amyloid, the biomarker of AD, prior to the onset of symptoms. The key difference between OAβ Test and other tests available on the market is that OAβ can selectively detect beta-amyloid oligomers.

OAβ Test Automated System
OAβ Test Automated System is an automated machine for measuring the oligomerization levels of beta-amyloid in individuals suspected of having AD.
Features
- Automatic quantitative and qualitative assay calculation and analysis of the result data
- Fully automated immunoassay system
- Zero carry-over by using disposable tips for liquid handling
- Level sensing for samples, reagents and washing solutions
- Serial pipetting to optimize the speed of the assay
- 8 manifold needles for fast washing
- Simple and intuitive User Interface
- Meticulous temperature control up to 42℃
| General | |
|---|---|
| Sample Capacity | 80 Patient Sample Positions |
| Reagent Capacity | 6 Custom Reagent Positions |
| Test Tube | Standard 13, 16mm diameter (Customizable) |
| Number of Microplates | 1 |
| Well-tracking | Independent well tracking & timing |
| System Architecture | Universal Open Platform (Qualitative and Quantitative) |
| Sample Tray | Easy loading and unloading of samples |
| Dimension | 650 x 830 x 650mm |
| Power | 100 - 240 V, 50-60 Hz |
| ELISA Reader Specifications | |
|---|---|
| Dynamic Range | 0 - 1,000,000 RLU |
| Photometer | Luminometer |
| Filter Slots | 6 |
| Washer | |
|---|---|
| Manifold Configuration | 8 channels |
| Dispense-volume Range | 100-300μl |
| Washing Tank | 2 External Washing Tanks and 2 Waste Tanks |
| Wash Cycle | 1 - ∞ (Customizable) |
| Residual Volume | <1μl |
| Incubator | |
|---|---|
| Temperature Range | Up to 42°C |
| Temperature Uniformity | ±0.5°C |
| Shaking | Amplitude: 1 mm, Speed: Up to 800 RPM |
| Temperature Monitoring | Yes |
| Pipetting | |
|---|---|
| Aspiration Volume | >5μl |
| Tip | Disposable |
| Serial Dilution | Yes |
| Replicates | Yes |
| Precision | <0.8% CV at 100 μl |
| Software | |
|---|---|
| Work Protocols (Assays) | Unlimited |
| Data Processing | Quantitative and Qualitative |
| Access Level Control | Yes (Master, Manager, and Operator) |
| O/S | Windows XP, 7, and 10 |